From Regeneration to Resistance: Comparative Insights into Platelet-Rich Plasma Therapy for Androgenetic Alopecia and Molecular Targeting in Glioblastoma Cell Strains


What is androgenetic alopecia?
Androgenic alopecia (AGA) often known as androgenetic alopecia or male sample baldness, is a standard dysfunction that impacts each women and men. Is likely one of the commonest causes for dermatological session worldwide.
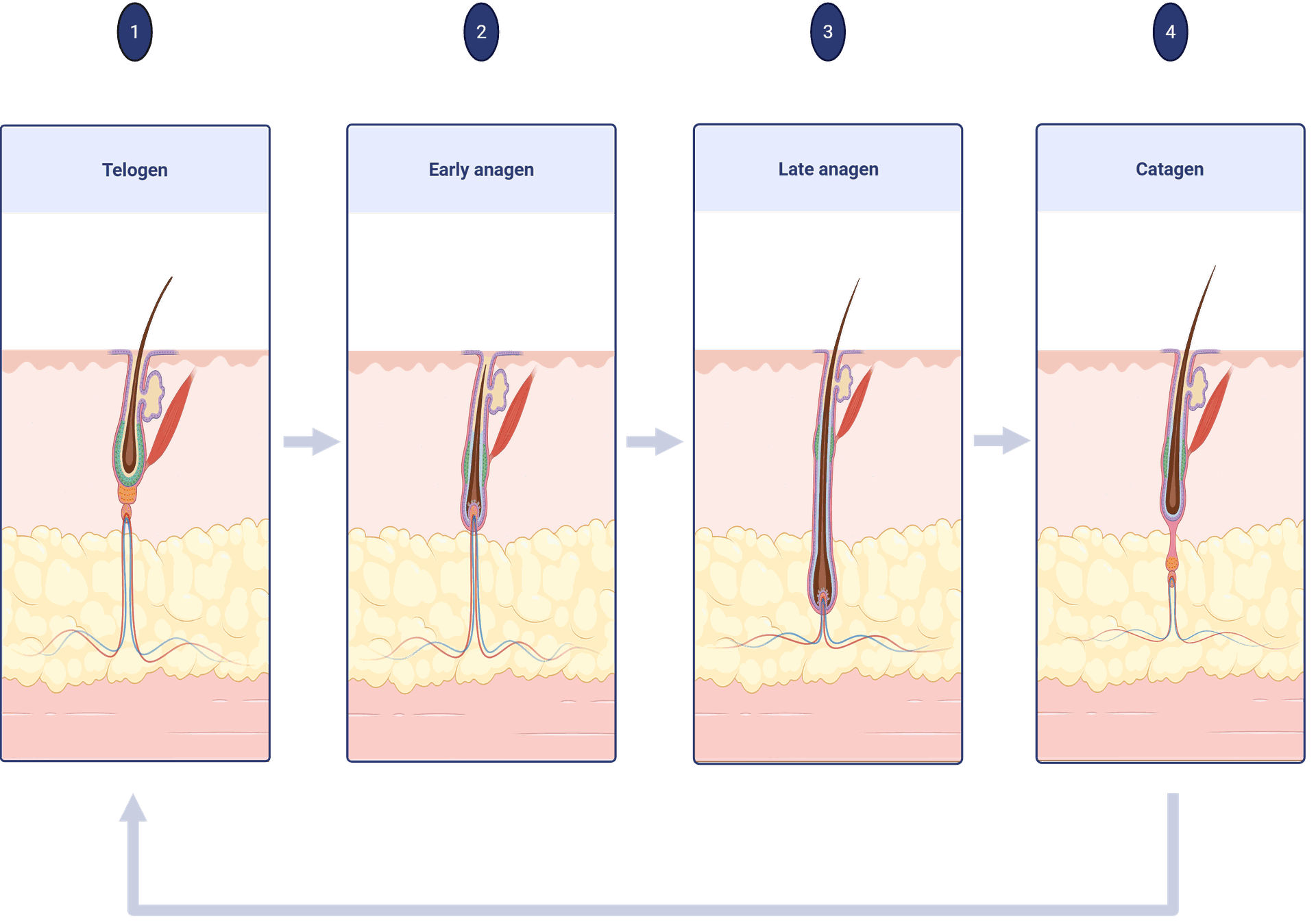
It’s characterised by progressive hair loss, particularly of scalp hair, and has distinct patterns of loss in girls versus males. AGA is an age-dependent dysfunction characterised by patterned hair loss. Based mostly on the few prevalence knowledge obtainable, Know that by the age of 30 years about 30% of males can have AGA and that this may rise to about 50% by the age of 50 years and as many as 90% of their lifetime , Though prevalence will increase with age in all populations, thinning can start as early as puberty.
The hair thinning begins between the ages of 12 and 40 years in each sexes and roughly half the inhabitants expresses this trait to some extent earlier than the age of 50 years . Androgentic alopecia is familial with a posh polygenic mode of inheritance .
Androgenetic alopecia, a common form of hair loss driven by hormonal and genetic factors, has long challenged both clinicians and patients seeking effective treatment options. Among emerging regenerative therapies, platelet-rich plasma (PRP) has garnered attention for its ability to stimulate hair follicle regeneration through localized delivery of autologous growth factors.
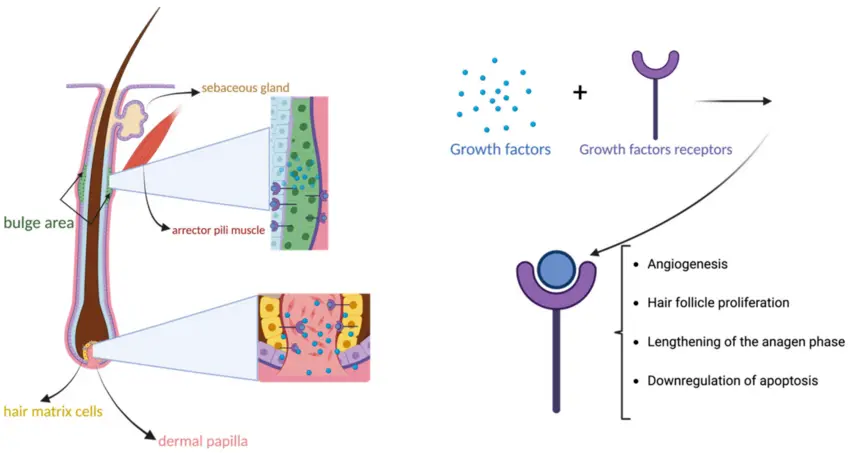
PRP is derived from a patient's own blood and contains concentrated platelets rich in bioactive molecules, such as platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), and vascular endothelial growth factor (VEGF). These factors promote:
- Angiogenesis around hair follicles,
- Cell proliferation of dermal papilla cells,
- And reduction of inflammation at the scalp level.
Systematic reviews and meta-analyses increasingly support PRP’s role in increasing hair density and thickness, particularly when administered in multiple sessions. Although the molecular pathways are still under investigation, the therapy appears to activate the Wnt/β-catenin signaling pathway, which is crucial for follicular development and cycling.
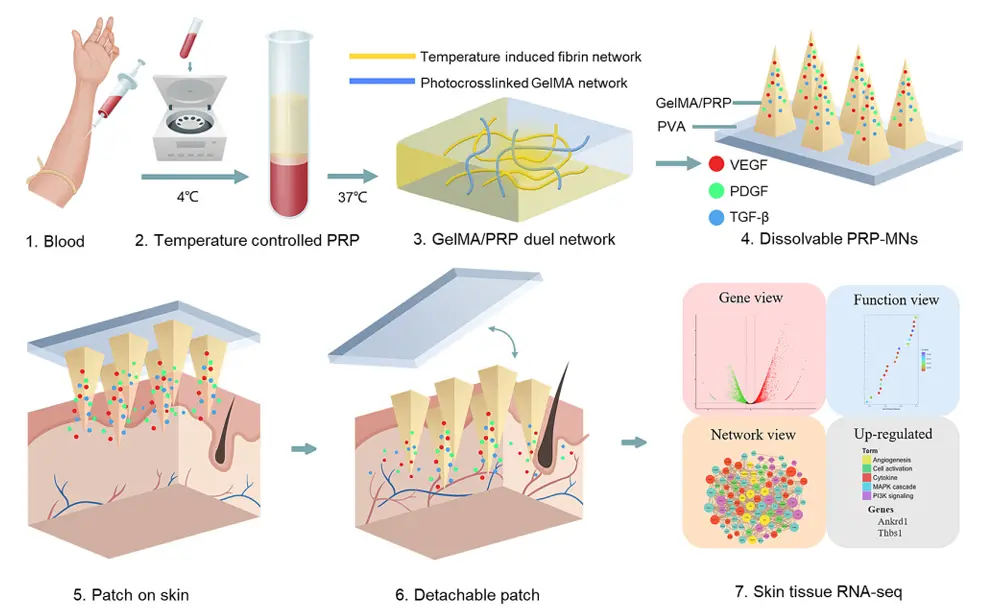
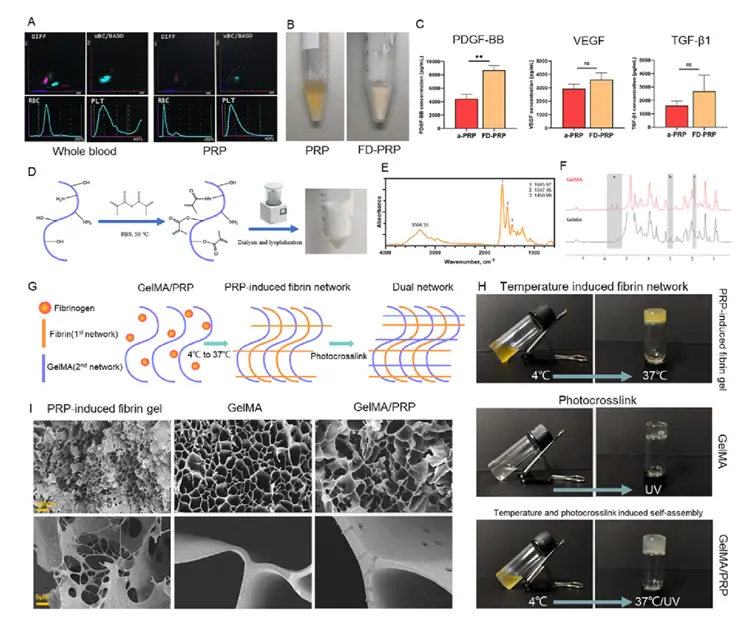
A temperature-controlled PRP-induced fibrin gel, interpenetrated with photocrosslinked gelatin methacryloyl (GelMA), represents a novel and promising sustained-release platform for hair regrowth therapy. This advanced biomaterial system leverages the biological activity of platelet-rich plasma (PRP)—rich in growth factors—while enhancing its stability and controlled delivery through fibrin matrix formation at physiological temperature. The incorporation of photocrosslinked GelMA adds mechanical strength and tunable degradation, enabling prolonged residence at the application site. Delivered via a base-detachable microneedle (MN) array, this system ensures minimally invasive and localized deposition into the scalp dermis, promoting effective hair follicle stimulation and angiogenesis. Together, the synergistic action of the fibrin-GelMA matrix and the sustained release of PRP bioactive molecules offers a powerful regenerative microenvironment for combating androgenetic alopecia and enhancing hair regrowth outcomes.
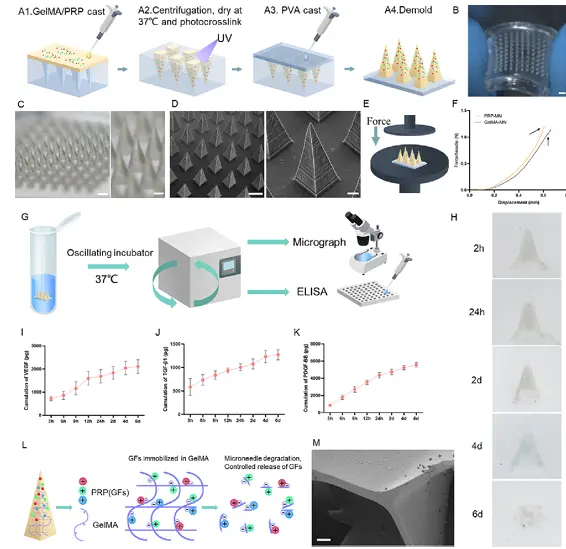
The fabrication process begins with the isolation of platelet-rich plasma (PRP) from whole blood via a two-step centrifugation protocol, enriching bioactive growth factors such as PDGF, VEGF, and TGF-β. To create a thermo-sensitive PRP-induced fibrin gel, PRP is combined with fibrinogen and thrombin precursors, allowing in situ fibrin polymerization upon exposure to physiological temperature (~37 °C), forming a soft, biodegradable matrix. Simultaneously, gelatin methacryloyl (GelMA) is synthesized through methacrylation of gelatin and dissolved in the PRP-fibrin mixture. Upon UV light exposure, the methacryloyl groups undergo photocrosslinking, creating an interpenetrating polymer network that enhances mechanical strength and degradation stability. The composite hydrogel is then characterized using scanning electron microscopy (SEM) to assess microstructure, rheological analysis to evaluate temperature-dependent gelation and viscoelastic properties, and release kinetics assays to measure sustained growth factor release. This dual-network matrix offers both biological activity and structural integrity, ideal for localized, long-term delivery in regenerative applications such as hair follicle stimulation.
Fabrication and in vitro characterization of PRP-MNs system
Glioblastoma Cell Strains: Decoding Resistance at the Cellular Level
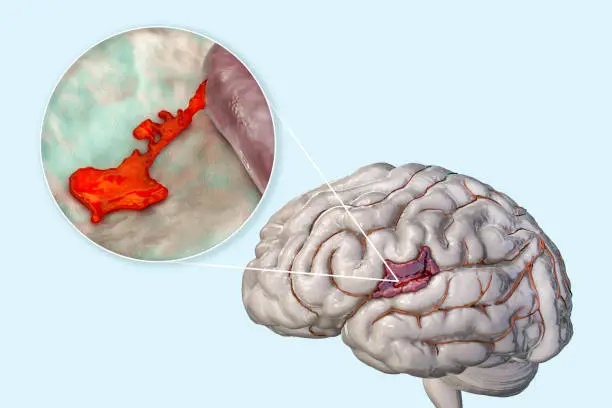
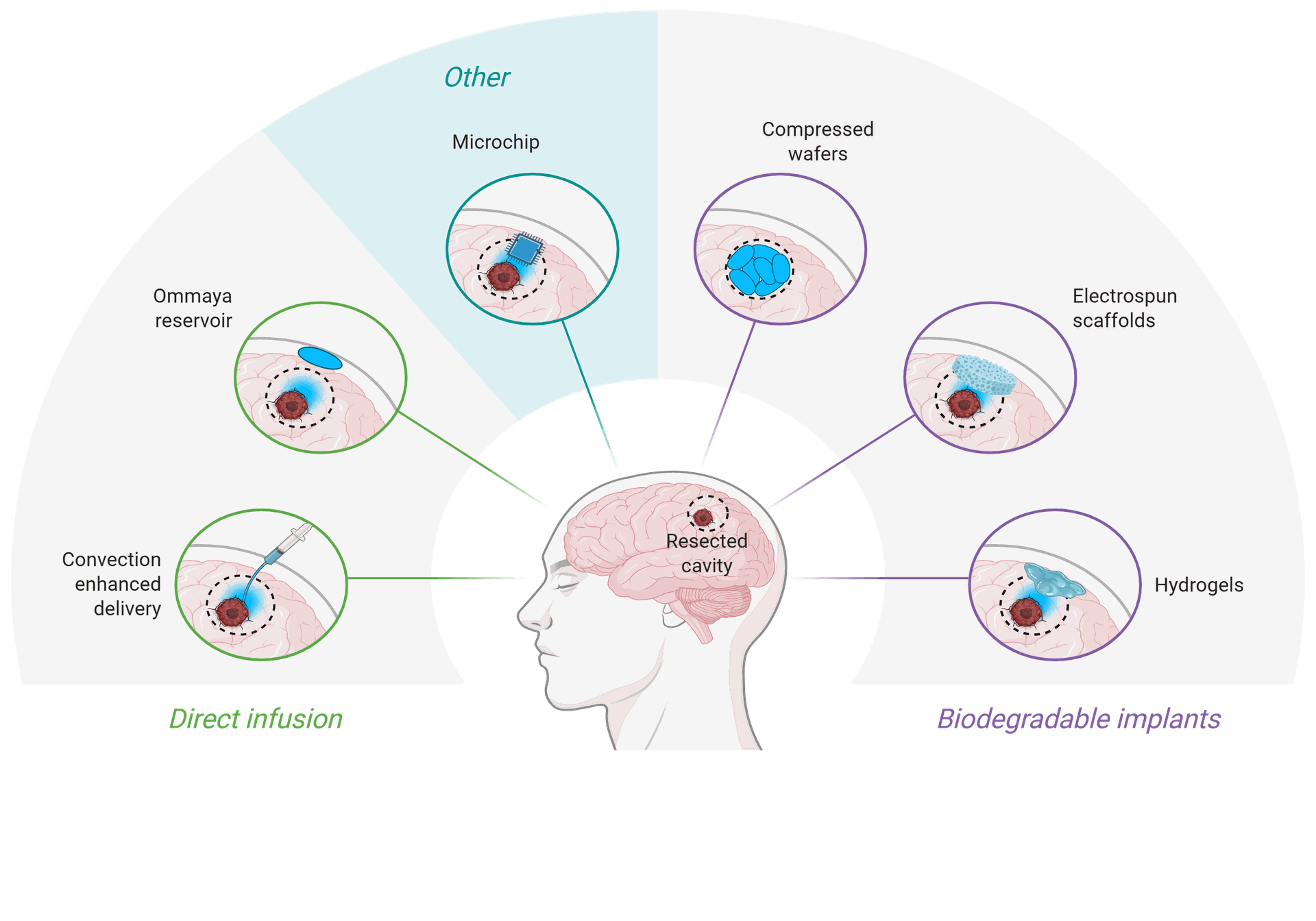
Glioblastoma (GBM) is probably the most aggressive kind of mind tumor arising from glial cells accounting for 52% of all parenchymal mind most cancers instances and 20% of all intracranial tumors. GBM has pronounced mitotic exercise, substantial tendency towards neoangiogenesis (microvascular proliferation), necrosis, and excessive proliferative charges. Due to their intrinsic infiltrative nature, GBM has a extremely aggressive malignant scientific course. The adjuvant chemo-radiotherapy (RT) with temozolomide (TMZ) after maximal protected resection stays the usual of care.
Mind most cancers is a novel due to the “blood-brain barrier”, which severely restricts the bloodstream of the mind. Whereas the blood mind barrier (BBB) is nice for shielding the mind from hazard, when the mind has most cancers cells, the BBB is usually a downside. Due to this fact, you will need to discover new drug targets.

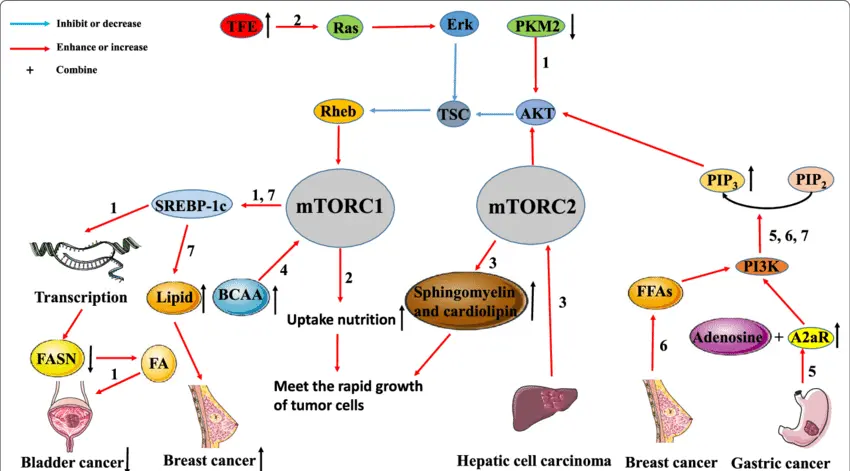
The upregulation of mTOR in GBM
The mammalian target of rapamycin (mTOR) signaling pathway is frequently upregulated in glioblastoma (GBM), contributing to the tumor’s aggressive growth, therapeutic resistance, and metabolic reprogramming. mTOR functions as a central regulator of cell growth, proliferation, survival, and angiogenesis, primarily through its two distinct complexes: mTORC1 and mTORC2. In GBM, mutations or aberrant activation of upstream regulators—such as PI3K, PTEN loss, or EGFR amplification—lead to persistent mTOR activation. This upregulation enhances protein synthesis, lipid biosynthesis, and inhibition of autophagy, facilitating unchecked tumor progression. mTORC2, in particular, promotes cytoskeletal organization and Akt phosphorylation, further supporting tumor cell migration and invasion. Therapeutically, mTOR has emerged as a potential target, but monotherapy with mTOR inhibitors (e.g., rapamycin analogs) has shown limited efficacy due to feedback loops and pathway redundancy. As a result, current strategies are exploring dual inhibitors, combinatorial therapies, and biomarker-driven approaches to more effectively disrupt mTOR signaling in GBM and overcome adaptive resistance mechanisms.
Anticipated outcomes of the scientific analysis challenge
Within the first occasion, the first consequence shall be to ascertain cell cultures and stem cells that faithfully reproduce in vitro the physiology of the tumor sustaining the identical traits of affected person’s neoplasm
1
Consider the impact of latest goal medication on the proliferation of major and steady human glioblastoma cell strains by organising development curves and methyl thiazolyl tetrazolium (MTT) toxicity assays.
2
Screening of pure and artificial medication utilizing patient-derived major glioblastoma cell strains
3
Characterize the mechanisms and proteins concerned within the apoptotic and / or autophagic pathway with immunohistochemistry and western blot assays in management and handled cells.
4
Validation of beforehand recognized molecular targets in preclinical fashions of mind cancers. The investigators are capable of determine novel molecular determinants that may be focused by pharmacological intervention to lower or block the tumor development.
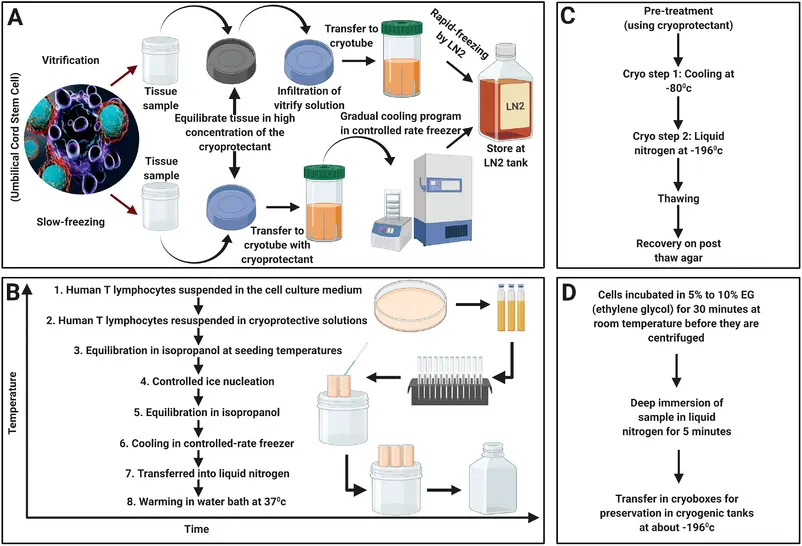
Tumor Specimen Assortment and Cryopreservation
Resection specimens of glioblastoma (GBM) tumors (n = 20) had been obtained sterile and freshly from Neuromed Neurosurgery. Tumor tissue samples had been snap frozen in liquid nitrogen and saved within the gasoline section above liquid nitrogen. Moreover, tumor tissue cubes (3 × 3 × Three mm) had been frozen vitally.
For this process, tumor items had been reduce with a sterile scalpel blade, and Four tumor items had been transferred right into a sterile cryo-tube in 1.5 ml freezing medium (fetal calf serum containing 10% DMSO), sealed in a freezing container (Nalgene, Rochester , USA), and positioned instantly at −80 ° C. Till thawing, tubes had been stored at −80 ° C (for at most of 6 weeks) or, after in a single day cooling, transferred to nitrogen tank (for longer storage durations).
Tissue Tradition and Cell Line Institution
With written consent from sufferers and/or in accordance with institutional tips, instantly after the resection gather tumor samples (200-500 mg of tumor is really useful) right into a tube containing chilly sterile stem cell media with out development components. Transport the specimen instantly to the tissue tradition hood for processing.
Immunohistochemistry
Consultant cell line of every tumour had been stained by Immunohistochemistry for Ki67(proliferation index estimated as a (%) proportion of constructive cells in a area of 100), IDH1, ATRX (markers of mind tumors) and GFAP (glial marker) (Ventana, Tucson, Ariz.) was carried out robotically with a Nexes instrument (Ventana). Antibody detection was carried out utilizing a multilink streptavidin-biotin advanced technique, and antibodies had been visualized by a diaminobenzidine chromagen technique. Detrimental management samples had been incubated with major antibodies solely.

Bridging the Concepts: Why Compare PRP and GBM Research?
At first glance, PRP therapy for AGA and molecular targeting in GBM appear to address opposite problems tissue regeneration vs. tumor suppression. However, they share a central theme: the manipulation of cellular microenvironments and signaling pathways to achieve desired biological outcomes.
Growth factor signaling
PRP leverages growth factors to rejuvenate tissue, while GBM often hijacks similar signals to fuel malignancy.
Therapeutic precision
In both cases, understanding individual molecular profiles (personalized medicine) is critical for optimizing efficacy and minimizing adverse effects.